[Updated Aug 14, 2021] Should I get the COVID-19 vaccine?
This post originally ran on December 14, 2020. Thanks to an overwhelming response by our community, we decided to make a major update to refresh the content after lots of great vaccine news in May 2021, and again in August.
Feeling a bit oversaturated by all the vaccine news lately? For people who are immunocompromised, live with an autoimmune or inflammatory condition, or are taking an immunosuppressant, the welcome news of the vaccine also raises concerns about how vaccines affect their immune systems, and how their medications might impact the vaccine’s effectiveness.
August 2021 update
As of August 14, 2021, here’s what we know:
The way that the COVID-19 vaccine works is by enabling your body to respond quickly and effectively when it encounters the SARS-CoV-2 virus. You can think of a vaccine like a seatbelt - while it doesn’t prevent your car from hitting another car, it blunts the effect of the crash on your body. The amazing thing is that these COVID-19 vaccines seem to blunt the crash very effectively, significantly reducing the risk of serious illness or death.
The latest data on the COVID-19 vaccine continues to show a very low risk of any serious complications, and it continues to be the best protection we have against COVID-19.
You are most likely to experience mild side-effects in the first few days after you receive the vaccine, and a very, very small number of people experience more severe side-effects in the first few weeks, including myocarditis, Guillain-Barre syndrome, and rare blood clotting disorders. (source)
There are no known long-term side-effects of the COVID-19 vaccines at this time, and the CDC is continually monitoring for these.
Your risk of long-term side-effects from COVID-19 is much higher than the risk you’ll have any serious issue with the vaccine. Many people who end up with COVID-19 do have long-term effects - recent estimates suggest about 10-20% of all people diagnosed with COVID-19 end up dealing with some form of “long COVID”. (source)
Yes, the vaccines work on the delta variant too! More than 90% of recent COVID-19 cases, hospitalizations, and deaths occurred in unvaccinated people (source). It’s important to note that there are studies with different estimates of exactly how effective the different vaccines are at preventing any symptomatic disease from COVID-19 - but in all studies published so far, even those focused on the delta variant, the current vaccines do significantly reduce your risk of symptoms, severe illness, and death.
Ages 12+: There is now a vaccine available in the US for everyone aged 12 and up.
Ages 5-11: Pfizer is expecting to submit an application to the FDA for use by the end of September; Moderna also expects to submit this fall. (source)
Ages 6 months - 5 years: Current estimates put the application submissions at the end of the 2021. (source)
Ages 0 - 6 months: Unclear as of yet. However, it does seem that when a mom is vaccinated, there are antibodies to COVID-19 present in the umbilical cord and in breastmilk - so infants may receive some protection in this way. (source)
There’s a new booster shot approved for people who are immunocompromised - see our recent blog post for more info.
If you have one of a few specific types of allergies, you may need to be careful about which vaccine you get. Check out this information from Yale Health to understand which vaccine is best for you.
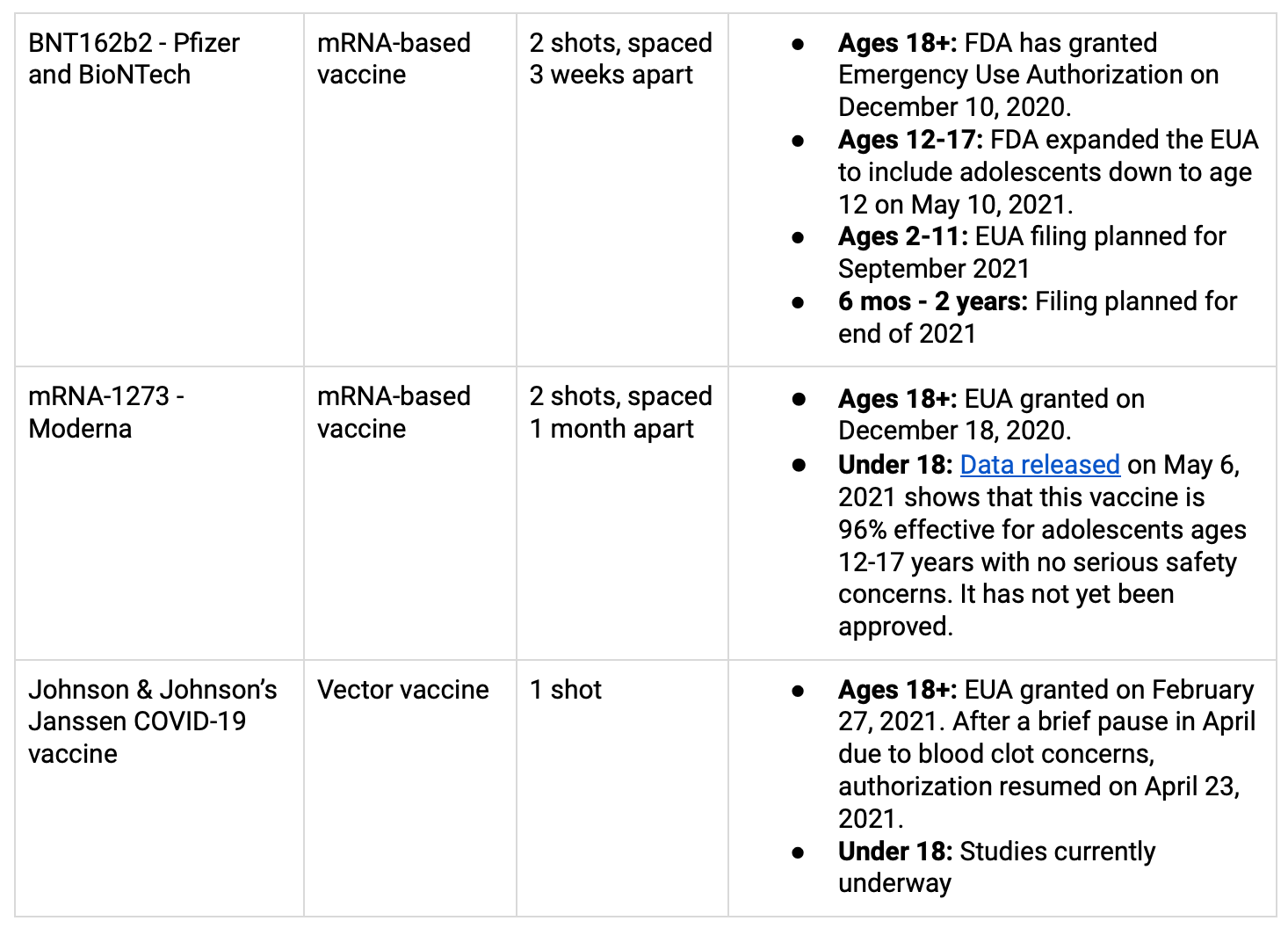
Question 1: What are the differences between the vaccines?
There are 3 types of vaccines for COVID-19 in the pipeline. All of them ultimately teach our immune systems how to recognize specific COVID-19 proteins, but in slightly different ways.
mRNA vaccines: Give our cells instructions (mRNA) for how to make a harmless protein that mimics the COVID-19 virus. These proteins then train our immune systems to remember it in the future.
Protein vaccines: Include pieces of COVID-19 proteins.
Vector vaccines: Include a weakened version of a live, less dangerous virus that has some genetic material from COVID-19 inserted into it. This “viral vector” then teaches our cells to make COVID-19 proteins.
The vaccines that are currently approved for use in the US (through an Emergency Use Authorization or EUA, by the FDA) can be found in the table below. More info on COVID-19 vaccines for children here.
Question 2: Should I try to get one vaccine over another type? What about the Johnson & Johnson vaccine and the blood clot concerns?
Experts are recommending that you receive any of the three vaccines that are available to you. They have all been approved for use by the FDA because they’re safe and effective. The differences in reported efficacy among the three vaccines all have to do with the likelihood that you’ll end up with mild or moderate COVID-19 disease after being vaccinated. The J&J vaccine reports efficacy of somewhere between 65-70% for mild/moderate, while Moderna and Pfizer report around 90-95%.
All three vaccines were highly effective (>85%) at preventing severe disease, and nearly 100% effective at preventing death from COVID-19. (More info)
There is a very, very small risk of blood clots (6 cases reported out of 6.8 million shots, as of April 12) from the Johnson & Johnson vaccine in women under 50 years old. If you are a woman under 50 years old and another vaccine is readily available to you, you might want to consider the other options. However, the CDC recommends that even for people in that category, the benefits of the J&J vaccine outweigh its known and potential risks.
You can find a very helpful Q&A from experts weighing in on the J&J vaccine here.
Question 3: What’s the deal with needing 2 shots? How much protection will I have after 1 shot?
That’s a great question! Short answer: Pfizer and Moderna both have strong effectiveness after one dose, but it’s been proven to increase after the second dose. The CDC is currently recommending that you go for both doses if you receive either the Pfizer or Moderna, so you get maximum protection. For J&J, there is an ongoing study to test whether adding a second dose will increase the effectiveness, but for now, everyone is receiving a single dose.
In the UK, to speed up the rate of vaccination, people have been receiving a single dose of vaccine, and then waiting a bit longer for their second dose. Even though this means they’re not getting the full effect, the UK has seen a dramatic decline in the number of new cases of COVID-19.
Question 4: What’s going on with vaccine side-effects? If I have a history of strong allergic reactions, should I be concerned? Should I take Tylenol before the shot?
According to the CDC, it’s uncommon to have a severe allergic reaction to any of the COVID-19 vaccines, but it can happen in individuals who have a history of severe allergic reactions or immediate reactions to vaccines and injectables. To make sure that you’re doing well, all people who receive a COVID-19 vaccine should be monitored for 15 minutes onsite. If you’re in the high-risk group, you should be monitored for 30 minutes.
About 1-2 people in every million will have a severe side-effect, but many people (in some reports, about 50%) will experience some discomfort. It’s common to have a more bothersome reaction after the second dose. These side-effects, which can include pain and swelling at the site of the injection, fever, fatigue, headache, and muscle/joint aches, are almost always not dangerous, and are more common in younger than older people. (More info here.)
Experts are recommending that you don’t take Tylenol or other pain relievers before you receive a dose of the vaccine, because it could actually cause your immune system to react less strongly. If you need a pain reliever after the vaccine, it’s okay to take one, but you should try to manage symptoms without it.
Question 5: What about variants? Do the vaccines work against different variants of COVID-19?
This has been a big and important question over the past several months! In mid-May, we started to see some very promising data - the World Health Organization now believes that the current vaccines are effective against current variants. This includes B.1.617, the latest variant which was first identified in India hand has spread to at least 26 countries.
The most important thing is for vaccination to be completed by as many people as possible. The more people who are vaccinated, the less likely that we are to see the emergence of new variants.
Question 6: Will the vaccine work if I have an immune deficiency or a compromised immune system?
This is a complicated question - it depends on the type of immune deficiency that you have, and the data is incomplete. For example, people with CVID are less likely to develop strong immunity from a COVID-19 vaccine, but those with Selective IgA deficiency may be more likely. There are new studies that will look at the effectiveness of the major vaccines in people with compromised immune systems. Stay tuned for those results!
Generally, the vaccine types that don’t use live virus, including mRNA vaccines and protein vaccines, are safe to take as a person with an immune deficiency. Many doctors recommend that you receive the vaccine, because it could boost your immunity somewhat. However, you may not develop as much protection as the average vaccine study participant, because your immune system may not respond fully to the vaccination.
The great news is - as soon as more people are vaccinated, there will be a higher level of COVID-19 antibodies available in plasma!
If you want to see how well you might have reacted to the COVID-19 vaccine, you can ask your doctor to order an antibody titer - even though this is not currently recommended by the CDC for most people. This test is not conclusive, but can give you some information about your response. We don’t know yet whether T-cell immunity or antibody immunity is more important for COVID-19 responses, but if you’re someone who is generally pretty good at creating T-cells, understanding more about your antibody response can be helpful information in assessing your overall COVID-19 risk.
PID UK has developed a very helpful vaccine fact sheet for the immune deficiency community. Find it here.
Question 7: I have another question! How can I get an answer?
Email us at [email protected] with the subject line, “COVID-19 Vaccine Question”. We’ll include your question in our next update! You can also leave a comment below.
Please note: this digest does not constitute medical advice, and you should consult your clinician if you have specific questions regarding your personal medical decisions.
Primary sources:
FDA advisory meeting, Dec 10, 2020
Pfizer Vaccine Ingredients (source):
The vaccine contains a nucleoside-modified messenger RNA (modRNA) encoding the viral spike glycoprotein (S) of SARS-CoV-2. The vaccine also includes the following ingredients: lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2- hexyldecanoate), 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-sn- glycero-3-phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose.
Moderna Vaccine Ingredients (source):
The Moderna COVID-19 Vaccine contains the following ingredients: messenger ribonucleic acid (mRNA), lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate trihydrate, and sucrose.
Johnson & Johnson Janssen Vaccine Ingredients (source):
The Janssen COVID-19 Vaccine includes the following ingredients: recombinant, replication-incompetent adenovirus type 26 expressing the SARS-CoV-2 spike protein, citric acid monohydrate, trisodium citrate dihydrate, ethanol, 2-hydroxypropyl-β-cyclodextrin (HBCD), polysorbate-80, sodium chloride.